Story by Bob Jones
Calcite, a calcium carbonate, is the most common of the carbonates. We admire its lovely crystal forms—all 600 of them. We also use it in huge quantities in its massive form, limestone.
Dolomite, a calcium, magnesium carbonate, is not as common as calcite. It also forms in lovely crystals, but not in as wide a variety as calcite. We also use dolomite in its massive form, dolostone. Which brings up two questions: “How do we distinguish calcite from dolomite?” and “How do we distinguish between limestone and dolostone?”
Distinguishing Traits
The first question is relatively easy to answer. Dolomite never forms in scalenohedral, or “dogtooth”, crystals, which is one of the common forms of calcite. Calcite also forms flat, rhomboid “poker chip” crystals, but dolomite does not. Calcite responds vigorously to acid, while dolomite responds less vigorously. Calcite is 3 on the Mohs Scale of Hardness, but dolomite is only Mohs 4-4.5. One other difference you may see is the smoothness of the crystal faces on your specimen. Dolomite crystals, because of an unusual internal structure, may have slightly curved crystal faces, while calcite does not. These are some simple tests you can use to determine the identity of a carbonate you own.

(irocks.com)
The second question is considerably more difficult to answer. Limestone and dolostone are both carbonate rocks, but externally there is little difference. The distinction really lies within the atomic structure of these carbonates. Scientists have a name for what’s happening: isomorphic substitution. Certain elements have the ability to substitute for each other in the atomic structure of a developing mineral’s molecules. This is possible because the atoms of different elements may be the same size, or nearly so. They may also have the same electron valence and atomic size to fit into a molecule’s structure. Calcium and magnesium are two such elements.
When calcium carbonate crystallizes, calcite crystals are the more likely result. But if enough of the element is present, magnesium atoms can substitute for some of the calcium atoms, and the result is calcium, magnesium carbonate, or dolomite.
This isomorphic substitution comes into play in limestone and dolostone. Limestone is the most common calcium carbonate rock. It forms on the floors of oceans, which are often made partly of the remains of shelled sea creatures. It is almost always somewhat impure, yet limestone is one of the major sedimentary rocks throughout the American Midwest. We quarry it, use it for road material, manufacture cement from it, cut it into building blocks, and use it for ordinary public buildings. Midwestern limestone is a tan or buff color, and is also commonly used in home construction.
Impact of Impurities
When limestone has few impurities, it is snow-white, or nearly so, and we use it for grave markers, monuments, and major public buildings, like the presidential memorials in Washington, D.C. When subjected to heat and pressure, limestone recrystallizes enough to form a harder rock with a sugary texture that we call marble. Artists love it as a carving material, especially when it is snow-white. So we tend to call sedimentary calcium carbonate rock with colors ranging from gray to tan to white limestone. But is it?
The problem is that calcium carbonate can be impure to the degree that it constitutes a different rock, dolomitic limestone or, as scientists now call it, dolostone. We have even named a whole mountain range in southern Europe the Dolomites!
The Dolomite Mountains are in the Tyrol region of Italy and are part of

(irocks.com)
the Italian Alps. They are of particular interest to mineralogists because this is where the mineral dolomite was first identified by D. Dolomieu. He recognized it as something unusual, as it was a carbonate that contained both calcium and magnesium. Further studies showed Dolomieu’s rock to be a new mineral. Both the rock and the mountains in which Dolomieu had obtained his samples were named after him.
During the development of calcium carbonate limestone, other elements are present and some of them get into the calcium carbonate structure. One of these is the metal element magnesium. It so happens that magnesium atoms are similar enough in size to calcium, only slightly smaller, and its electron valence matches that of calcium. This means magnesium can substitute for, or take the place of, some of the calcium in the molecular structure of the limestone. It actually bonds within the molecular structure, becoming a permanent part of the structure. This differs from elements that are simply impurities trapped in the limestone rock.
Transition to Dolostone
When enough magnesium substitutes for calcium in the limestone, the rock is no longer limestone. We now call it dolostone. The term dolomitic limestone has been in use for a long time and is still used for impure forms of limestone. The problem is that you can’t visually distinguish between limestone and dolostone.
Anyone who has collected the New York quartz crystals known as Herkimer Diamonds has had to deal with the hard, gray rock in which the quartz is found. We used to call that gray rock dolomitic limestone, but the 500 million-year-old rock is properly referred to as dolostone today. If you have worked the dolostone around Middleville (Herkimer County), New York, you know that it is somewhat harder than most limestone and is slightly less affected by acids.
Dolostone is not to be confused with dolomite, a true mineral. The calcium, magnesium carbonate develops in fine rhombohedral crystals. This dolostone formed in relatively shallow seas, in which the ancient life form called stromatolites could develop.
Stromatolites are credited with producing a lot of the atmospheric oxygen that made the development of other life forms possible. During the development of the Herkimer County dolostone, stromatolites lived and died, leaving openings in the dolostone in which later silica solutions created the water-clear, doubly terminated quartz crystals we seek.
Common Case Atom Substitutions

(Wikipedia)
This business of isomorphic substitution of atoms (one element substituting for another element in the structure of a rock or mineral) is common. In the case of limestone and dolostone, the magnesium atoms substitute for calcium. Keep in mind that limestone forms on the ocean floor and can be virtually pure calcium carbonate. But it is not uncommon for later solutions that are rich in magnesium to infuse into the limestone and alter its composition to dolostone.
There are a number of metamorphosed limestone or marble deposits in America that are pure enough to be used for special purposes. A few such deposits are well known to us. Fine limestone has been quarried in Vermont since colonial days. Superb white marble has been quarried in Carrara, Italy, since ancient Greek (510-323 BCE) and ancient Roman (753 BCE–476 AD) times. It was used to build the Parthenon in Athens (circa 447 BCE) and the Taj Mahal in India (circa 1632), for example.
High in the Rockies, at Marble, Colorado, is the Yule Marble quarry, which supplied the marble blocks used in the Lincoln Memorial and the Tomb of the Unknowns in Washington, D.C.
The tectonic movements of the earth can be so dramatic that it can lift ocean-bottom limestone thousands of feet. The evidence is in the upper layers of rock on Mount Everest, where fossilized limestone has been found on the peak!
Chemical Similarities
The minerals calcite and dolomite are chemically similar and actually form a series ranging from pure calcium carbonate calcite to pure calcium, magnesium, carbonate dolomite. The magnesium atoms that substitute for the calcium are a bit smaller than those of calcium, and this affects the appearance of many dolomite crystals. Dolomite crystals can show this atomic size difference externally as a slightly curved surface. When the curve is pronounced, the crystals take on the shape of a saddle. These can be seen in the Niagara Escarpment limestone, a formation that runs through New York, Ontario, Michigan, Wisconsin and Illinois, and are common in the Tri-State district of Missouri, Oklahoma and Kansas.
The curvature seen in small dolomite crystals is interesting. It results from the internal atomic structure. The slightly smaller size of the magnesium atoms that substitute for calcium atoms offsets the internal molecular structure, decreasing its symmetry. This is expressed externally as a curved surface. It can also give larger dolomite crystals a slightly rough or uneven surface.
Other elements besides magnesium can get into the structure of some carbonates. If there is enough iron in the structure the mineral ankerite develops. This is an iron, magnesium, manganese, carbonate. In some ways, ankerite looks like dolomite and forms a series with it. If iron, magnesium and manganese all join in the carbonate structure, another species is formed: kutnahorite, a calcium, manganese, magnesium, iron carbonate.
It can form a series with ankerite.
This same substitution happens in other minerals. One example is when cobalt and cadmium substitute for each other. When cobalt replaces calcite, it gives the mineral a lovely pink color, and we call it cobaltocalcite. Cadmium can substitute in smithsonite, giving it a nice yellow color.
Dolomite crystals do not develop as often as calcite, which is by far the most abundant carbonate mineral. But dolomite does form fine crystals that can be very showy. It is also often seen as an accessory mineral associated with larger and more popular mineral species.
Rhomb Leads Common Crystals
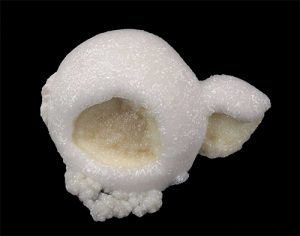
The most common crystal form of dolomite is the rhomb. These can grow up to several inches across, though small, slightly curved, or saddle-shaped crystals are more common.
Most dolomite is white to slightly gray, but it can be light- to dark-
(irocks.com)
brown or pink. The brown color results from the presence of iron, again substituting for other atoms in the structure. The greater the substitution involved, the darker the tan to brown color. The pink color seen in some dolomite results from manganese substituting in the structure.
Dolomite is found in a variety of places. In recent years, it has been found in superb crystals associated with other very fine species in the Shangbao mine, in China. Specimens of dolomite with superb, associated fluorite are exceptionally fine. In a few cases the dolomite rhombs actually exceed in size the associated fluorite cubes.
Pakistan has also been producing excellent example of dolomite at Turniq, near Shingus. Africa has a couple of localities that are well worth remembering. The Democratic Republic of Congo has been producing delightful cobaltodolomite crystal groups with a lovely purplish-pink color. These are very showy and well worth owning. Zaire is also good source for similar cobaltodolomite specimens in tightly clustered groups of fine, small crystals.
The color of these dolomites makes them among the most popular dolomites available today. The huge quarry at Bruamdo, Bahia, Brazil is best known for magnesite crystals of excellent size and quality. Associated with the magnesite are water-clear optical dolomite rhombs, some of which exceed 6 inches on an edge. These are probably the finest dolomites available, though in very limited numbers, on the market today.
Dolomites Dominate Market
In the 1970s, superb dolomites from Eugui (also Eugi), Spain, appeared on the market. These remarkable specimens were mined by just one adventuresome fellow, Victor Yount, a very well-known dealer who risked life and limb to collect minerals. To collect aragonite cluster groups in Spain, he had his friend hold his ankles while he hung over the edge of a cliff. To collect the amazing vanadinite specimens from Morocco, Vic braved the desert of Morocco and encounters with Bedouin tribes and the Moroccan police.
Collecting the Eugui dolomites was another adventure undertaken by a truly remarkable collector. Incidentally, he also ran with the bulls in Spain and appeared as an extra in several well-known movies, including “Patton”, which was shot in Spain.
Some of the prettiest dolomite crystals occur in the deep gold mine at Morro Velho, Brazil. The dolomite crystals are curving (sometimes called “saddle-shaped”), gemmy beauties associated with stunning pink fluorapatite. They tend to grow to about a half-inch to an inch across, but are arranged in nice, freestanding clusters that are very attractive.
Dolomite often occurs associated with other more attractive minerals, so it is sometimes overlooked, but it can be very attractive and certainly deserves a place in everyone’s mineral collection.