By Steve Voynick
Medieval alchemists failed in their attempts to transmute common materials into precious ones. But as it turned out, their visions of transmutation, especially regarding rubies and sapphires were valid, albeit centuries ahead of their time.
The scientific feasibility of synthesizing rubies and sapphires did not become apparent until the late 1700s after mineralogists realized that these gemstones consisted not of mystical materials, but of certain forms of the mineral corundum (aluminum oxide, Al2O3).
The first step in creating rubies and sapphires came in 1817 when French chemist Joseph-Louis Gay-Lussac synthesized corundum by heating ammonium alum (hydrous ammonium aluminum sulfate). France then became a hotbed of research into corundum-gemstone synthesis. Chemists soon learned that pure aluminum oxide is colorless, but that traces of chromium impart the red color of ruby, while those of iron and titanium provide the blue of a classic sapphire.
Chemistry Transforms Corundum
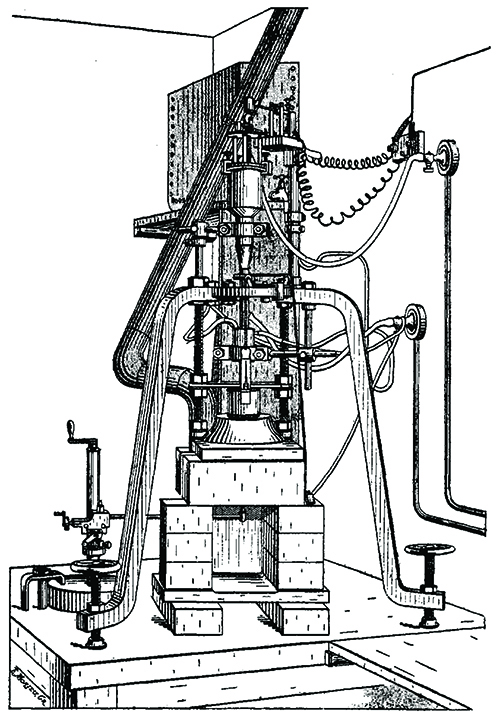
Verneuil used in 1907 to synthesize ruby.
In 1837, Marc Antoine Gaudin synthesized a non-gem ruby by adding chromium oxide to pure aluminum oxide, then slowly crystallizing the molten mix. A decade later, researchers synthesized tiny sapphire crystals with a similar flux process.
By 1866, French chemists had developed a flame-fusion method of ruby synthesis by dropping particles of aluminum oxide and chromium oxide through a hot oxy-hydrogen flame. The molten particles collected below the flame and crystallized as tiny crystals of synthetic ruby. A succession of flame-fusion and flux experiments followed. While the results were sometimes encouraging, none produced synthetic gemstones large enough to facet and inexpensive enough to underprice natural gems.
Finally, in the 1890s, French chemist Auguste Victor Louis Verneuil commercialized flame-fusion synthesis of gem-quality ruby. Verneuil mixed pure, powdered aluminum oxide with chromium oxide then, while carefully controlling time, motion, and temperature, passed the particles through a stream of oxygen into a hot gas flame. The molten droplets crystallized on a slowly revolving rod as a cylindrical “boule” of transparent, gem-quality, synthetic ruby.
Growing Rubies
Initially, Verneuil needed three hours to “grow” a 15-carat boule, each cuttable into eight carats of faceted gems identical in appearance to those of pricey, natural ruby, but far less expensive. In 1907, when Verneuil was growing two-foot-long ruby boules in eight hours, he opened a commercial laboratory and was soon synthesizing two tons of gem-quality ruby annually. When millions of carats of “Verneuil ruby,” then indistinguishable from natural stones, reached European jewelry buyers, natural ruby prices plummeted and a financial panic rocked the gem markets.
Verneuil then began synthesizing flame-fusion sapphire using iron and

process; the sapphire and ruby by the flame fusion (aka Verneuil proces). GRENDELKHAN, WIKIMEDIA COMMONS
titanium oxides as blue chromophores—and promptly catapulted the sapphire markets into panic. The markets and prices finally stabilized following World War I when gemologists learned to recognize the subtle “growth-ring” swirls of microscopic gas bubbles that characterized Verneuil’s flame-fusion corundum gems.
World War II redirected the focus of flame-fusion ruby synthesis from gems to scientific and industrial applications, mainly the manufacture of millions of tiny, mechanical jewel bearings for such precision instruments as chronometers, bombsights, and optical range finders.
Evolution of Flame-Fusion
Following the war, companies began synthesizing flame-fusion “star” rubies and sapphires by mixing aluminum oxide with red or blue chromophores and powdered rutile (titanium dioxide). The rutile created both translucency and a reflective, six-rayed “star” pattern along the lateral axes of the hexagonal corundum crystals. Synthetic star rubies and sapphires cut as cabochons became wildly popular in jewelry, while transparent, synthetic corundum gems were—and still are—faceted and set into millions of high-school and college graduation rings each year.
was in mechanical jewel bearings of fine watches and other precision instruments.
Gemologists can now easily distinguish synthetic flame-fusion rubies from natural rubies, giving both natural and synthetic stones clearly defined places in the gem markets.
Auguste Verneuil would undoubtedly be pleased to know that most synthetic rubies and sapphires are still created through the flame-fusion process that he commercialized more than a century ago—as would the old alchemists to know that their dreams of transmuting common materials into precious gemstones—albeit synthetic—weren’t so far-fetched after all.